A Molecule That Is Sp3d Hybridized and Has
HC C C. Which of the following statements correctly defines hybrid orbitals.

Solved 17 A Molecule That Is Sp3d Hybridized And Has A Chegg Com
-VB theory predicts that the bond angles in PF3 will be close to 900.

. Give the hybridization states of each of the carbon atoms in the given molecule. Preface The Essentials of Physical Chemistry has been written for BSc students. It has been national bestseller for more than 65 years.
So this makes the atom excited and the configuration now has 5s2 5p5 5d1. The bond angles for this molecule are slightly less than 1095o. H 2 C CH CN.
If there are only four bonds and one lone pair of electrons holding the place where a bond would be then the shape becomes see-saw 3 bonds and 2 lone pairs the shape is T-shaped any fewer bonds the shape is then linear. The Lewis structure for the molecule NF3 is shown. The shape of the molecule can be predicted if the hybridization of the molecule is known.
Orbitals used for bonding that are. The N lone pair is located in an unhybridized p orbital. The bigger lobe of the hybrid orbital always has a positive sign while the smaller lobe on the opposite side has a negative sign.
The N atom has three bonds and is therefore sp2-hybridized. Select all the statements that correctly describe the bonding in the molecule PF3 in terms of valence bond theory-Each P-F bond is formed by the overlap of a 3p orbital from P with a 2p orbital from F. In the case of BrCl3 having 3 bonding pairs and 2 lone pairs around the central atom will correspond to a steric number of 5.
Select all the statements that correctly describe the bonding in this molecule. It has been used by more than 2 million students. It really has been that long.
PF3 has back bonding in which fluorine donate its lone pair of electron to the vacant d orbital of phosphorus atomDue to back bonding higher bond pair bond pair repulsion PF3 higher bond angle than PH3. Enter the email address you signed up with and well email you a reset link. The geometry of the molecule will thus depend on the number of bonding as well as non-bonding electrons present on the central atom.
For sp3d hybridized central atoms the only possible molecular geometry is trigonal bipyramidal. In this case it would suggest us an sp3d hybridization. So the hybridization here is sp3d.
Now we have to find out which AO combined with the other AO to form hybridized orbitals. Answer 1 of 5. If all the bonds are in place the shape is also trigonal bipyramidal.
It is 26 editions old. Now here xenon has more than eight electrons in its valence shell unpaired as per Lewis Structure. Two hybrid orbitals are used for sigma bond.

Image Result For Hybridization Sp Sp2 Sp3 And Shape Molecular Shapes Chemistry Chemistry Help
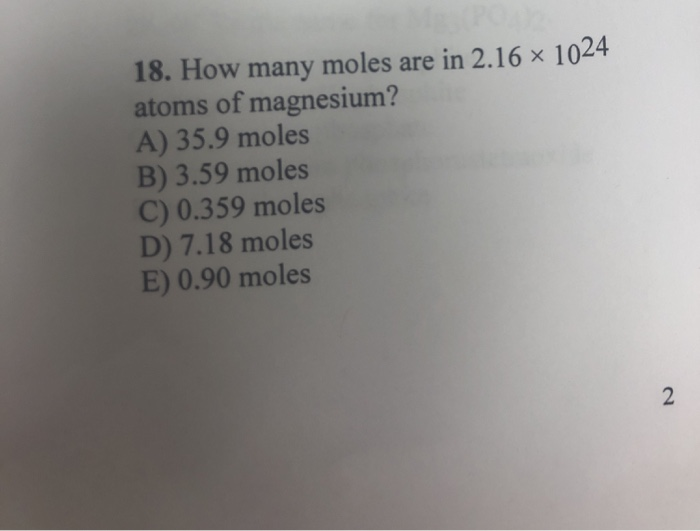
Solved 17 A Molecule That Is Sp3d Hybridized And Has A Chegg Com


Comments
Post a Comment